PredictOR – Carbon Analytics
 Driving for the NHS Net Zero target in surgery
Driving for the NHS Net Zero target in surgery
TCC-CASEMIX is one of 10 leading edge health tech businesses selected in competition from across the UK to produce a proof of concept to demonstrate how the NHS could deliver Net Zero performance. TCC-CASEMIX’s PredictOR vision was selected to deliver a decision-support system for patients and surgeons informed by the both medical device performance and the carbon impacts of different surgical devices and surgical options.
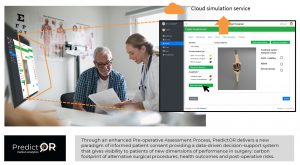
PredictOR will deliver a significant new resource to enable patients, surgeons and anaesthetists to make better informed decisions concerning medical device performance – not just the functional performance relative to the patient needs, but the carbon performance too.
Deployed through our Open Registry Infrastructure, presented below, PredictOR will provide a unique repository of medical device performance across Europe. Further added value will be that this infrastructure will support a new era of value-based procurement of medical devices.
The development work is being delivered in partnership with members of the ABHI. The University of Plymouth, and Royal Cornwall Hospitals NHS Trust are also supporting the research.
TCC-CASEMIX
Medical Device Information System – Post-Market Surveillance – Open Registry Infrastructure.
Developing a Medical Device Information System [MDIS] has been a central ambition of TCC-CASEMIX since we commenced trading in January 2020. Thanks to a significant investment by UK Research & Innovation this is now a reality and already commercially deployed. Our significant European deployment has been in Szeged, Hungary, in the Department of Orthopaedics and Traumatology, led by Professor Varga. We are currently [March 2022] managing enquiries from Netherlands, Belgium, and Austria.
The next step in the development of the MDIS, has been to establish the infrastructure for Post-Market Surveillance [PMS] of medical devices used in surgery. This enables medical device manufacturers to demonstrate compliance with Medical Device Regulations in both UK and mainland Europe. To achieve this major innovation, TCC-CASEMIX has developed the foundational work for an Open Registry Infrastructure. This infrastructure is the means to receive PMS data streamed real-time data from the MDIS. Our first deployment is now being developed in the South-West of England
The significance of the Open Registry Infrastructure cannot be underestimated, because it enables medical device registries receiving data at hospital level to be automatically aggregated into regional registries, and then into national regsitry, thereby enabling manufacturers to ‘harvest’ performace data relating to their devices from across the registry infrastructure. Conventional practice makes this very hard to achieve – both expensive and time-consuming for device manufacturers.

Follow us
on Twitter