Digital Pathways
Click on image to enlarge
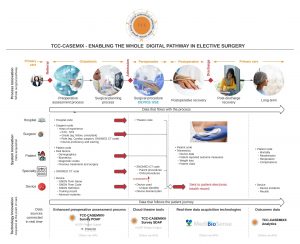
1) A digitally enabled surgical pathway
Data flow into TCC-CASEMIX commences with receipt of a partial electronic patient record [FHIR compliant] containing demographic and diagnostic data [coded in SNOMED CT].
Within TCC-CASEMIX the patient data is mapped to specific risk factors that impact surgical complexity. Engaging with the patient in an enhanced preoperative assessment process, these risk factors are then mapped to the recommended surgical procedures. These too are pre-coded in SNOMED CT. This has been achieved by a real-time data feed from the SNOMED CT servers. There is now a complete data trail from patient presentation in the Primary Care setting, into the operating theatre of the Acute Care setting, all joined through a common data structure.
Ultimately this data trail can then be extended into a digitally enabled post-operative care setting when the patient returns home. Patient Reported Outcome Measures [PROMS] further enhance the patient record, achieved with our partner Medibiosense, and their unique wearable technologies. https://www.medibiosense.com/
On completion of surgery a FHIR compliant record is then returned to the customer with the enhanced electronic record detailing the surgical interventions that were undertaken. This is the true embodiment of a digitally enabled surgical pathway.
2) A whole new surgical dataset
A Surgical Data Acquisition Process. Procedure duration data is harvested in real-time from the operating theatres that deploy TCC-CASEMIX. A machine learning algorithm maps patient risk profiles to the impact on measured surgical procedure durations. It is this capability that drives statistically predictable forecasts of procedure duration for theatre list planning. Achieving these new levels of predictability is the essential prerequisite to improving productivity in surgical services delivery.
3) A Medical Device Information System
Another innovation in TCC-CASEMIX is to enable any medical device to be identified from a register of devices and ‘tagged’ to a surgical procedure. The significance from a digital pathway perspective is that each device is identified by its UDI and GMDN code and then mapped to a surgical procedure through a corresponding SNOMED CT code. By this means TCC-CASEMIX provides full traceability of medical devices, and accordingly enables full Post-Market Surveillance [PMS] as required by the Medical Device Regulations in the UK and mainland Europe.
4) An Open Registry Infrastructure with a Data Access Portal.
With each deployment of TCC-CASEMIX the customer will eventually receive a free connection to a specialty specific, medical device registry. The Open Registry Infrastructure along with the Data Access Portal, are the subject of a UK Research & Innovation project. We expect this service to be available during 2022.
It is this major innovation that will provide complete transparency of medical device performance and heralds a new era of patient safety and patient informed consent.

Follow us
on Twitter